Speaker
Description
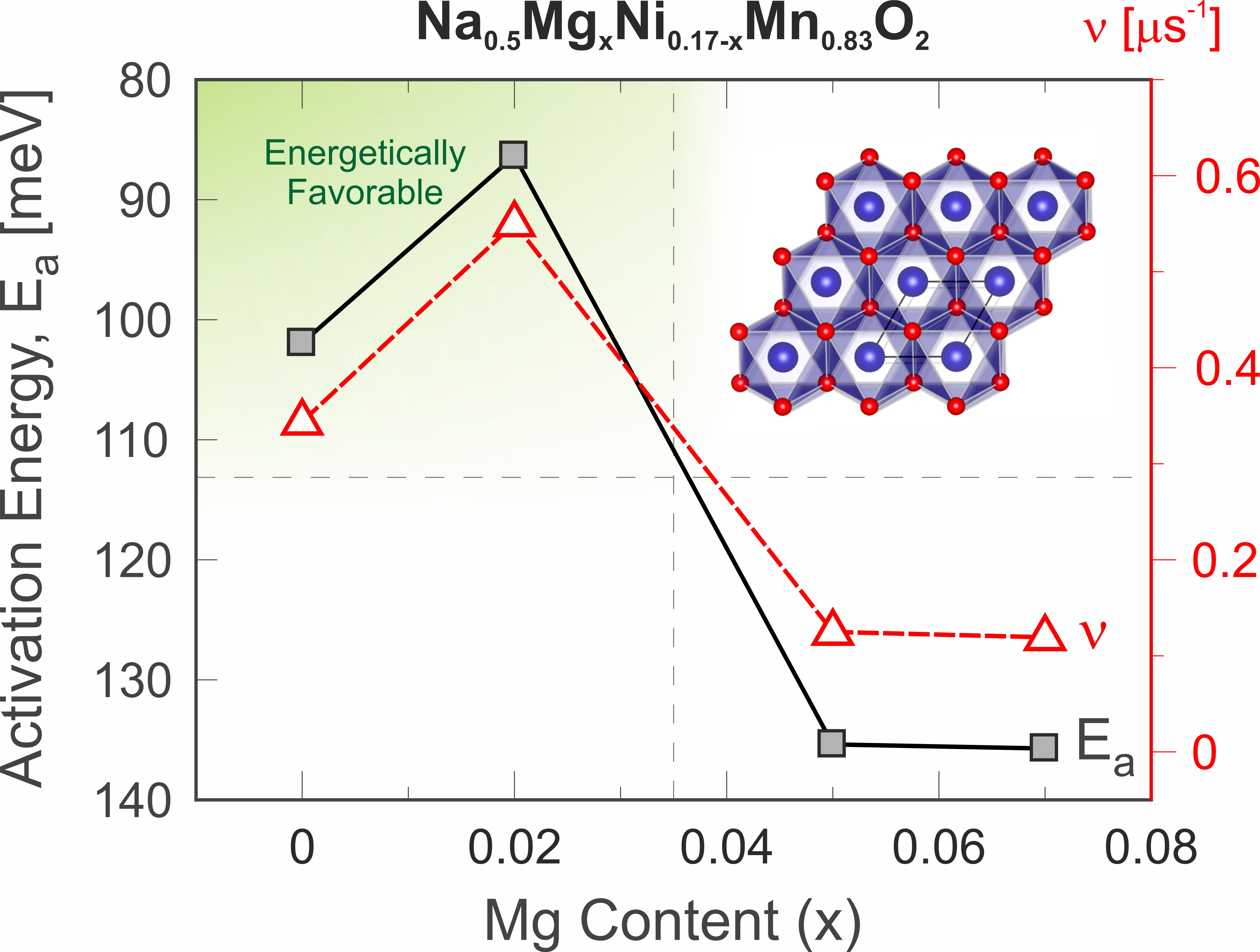
While Li-ion batteries are considered the main candidate for mobile applications, compounds based on lithium’s heavier cousin, sodium (Na) have also started to receive a lot of attention lately as candidates for future batteries. One reason is that the Li-reserves are limited and if large scale energy storage become a reality in our future sustainable society, we might have to consider alternatives to the Li-ion technology [1]. During last decade, an increasing number of new Na-battery materials with improved performance have been discovered and the general interest for sodium-based energy storage have increased tremendously. Among the cathode materials the 2D layered P2 – Na$_{2/3}$Ni$_{1/3}$Mn$_{2/3}$O$_{2}$ compound [2,3] has shown promising storage capacity and operating voltages above 3.5 V. Unfortunately, this material displayed very poor cyclability i.e. short battery life times, directly related to structural transition during the charge cycles. A potential remedy was found by partly substituting Ni for Mg. The resulting Na$_{0.67}$Ni$_{0.3−x}$Mg$_{x}$Mn$_{0.7}$O$_{2}$ compound [4] also displayed improved Na-ion diffusion rates. In this study we have investigated the Na-ion self-diffusion by means of muon spin rotation ($\mu^+$SR) [5,6] for the compound series $0\le x \le 0.07$. We surprisingly find that even a very small amount of Mg substitution (x = 0.02) results in the best cycling stability and highest Na-ion mobility [7].
[1] G. Alexander, J.B. Goodenough, M. Månsson, et al., Physica Scripta 95, 062501 (2020)
[2] Z. Lu, et al., J. Electrochem. Soc. 148, A710 (2001)
[3] Z. Lu, et al., J. Electrochem. Soc. 148, A1225 (2001)
[4] G. Singh, et al., Chem. Mater. 28, 5087 (2016)
[5] Sugiyama, Månsson, Phys. Rev. Lett. 103, 147601 (2009)
[6] M. Månsson & J. Sugiyama, Phys. Scr. 88, 068509 (2013)
[7] Le Anh Ma, et al., Physical Chemistry Chemical Physics 23, 24478 (2021)

